Abstract
Background
Patients with established cancer diagnoses often experience delays in starting scheduled inpatient chemotherapy (chemo) after arrival to the University of Virginia Medical Center oncology unit. These delays not only compromise the quality of individual patient care but also negatively impact hospital resource utilization, length of stay and delay other patient admissions.
Methods
To investigate these delays, we formed a multidisciplinary team of physicians, nurses, and pharmacists. Retrospective chart review was done and of the 340 planned chemo admission encounters in 2015, 100 were randomly reviewed to establish our baseline dataset. For each of these encounters, extensive patient demographic, clinical, and inpatient event data were collected and analyzed to determine time from patient arrival to start of chemotherapy (TTC). In the absence of national guidelines on TTC, we aimed to decrease TTC by 10%. With guidance from the American Society of Clinical Oncology's Quality Training Program, our team utilized multiple qualitative and quantitative tools to further understand factors contributing to TTC delays. These tools included a process map and Ishikawa cause-and-effect diagram to depict all the steps in between patient arrival on the inpatient oncology unit and the start of chemo, a Pareto chart to quantify delays of individual steps, and a priority matrix categorizing potential interventions based on impact and ease of implementation. XmR charts, a type of quality improvement control chart, were used to depict TTC and length of stay (LOS) of the baseline population and intervention groups.
Results
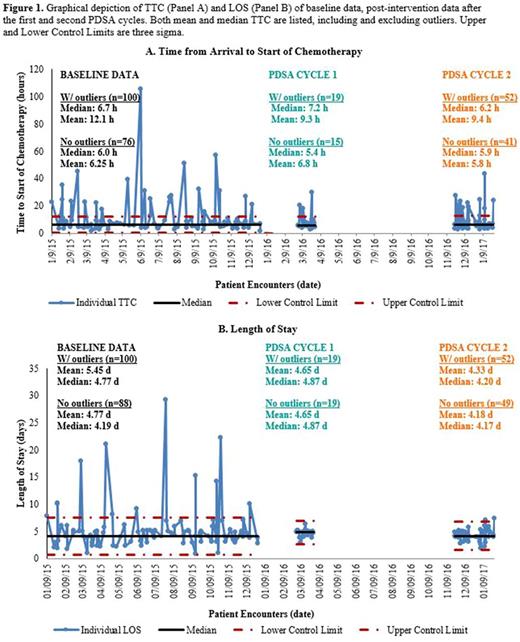
In the baseline population, median TTC was 6.7 hours (h) and mean TTC was 12.1 h. The disparity in these numbers indicated the existence of extreme outliers in our population, most of which were due to urine parameter requirements. Two Plan-Do-Study-Act (PDSA) cycles of interventions were implemented and post-intervention data were collected and analyzed. The first PDSA cycle involved reforming the chemo consent process. Previously, paper forms were used for informed consent, but these forms were often lost and not scanned into the electronic medical record (EMR). When missing, chemo was not started until a physician obtained informed consent on a physical form again. This PDSA cycle involved electronic and searchable documentation of risk-benefit discussions regarding chemo in the EMR. Unfortunately, median TTC after PDSA cycle 1 (19 encounters) was unchanged at 7.2 h. Median LOS was also unchanged (4.87 days (d) in PDSA cycle 1, compared to 4.77 d at baseline). The chemo consent change, however, remained in place for the convenience of patients and physicians. A second PDSA cycle involved routine pre-review of the inpatient chemo treatment plans by a clinical pharmacist one business day prior to the patient's planned admission. This review was based on a standardized checklist of questions involving the admission, specific chemo regimen and parameters required. The XmR chart for PDSA cycle 2 showed a reduction in median TTC from 7.2 h to 6.2 h (52 encounters). This met the team's goal of improving TTC by 10%. Additionally, median LOS improved from 4.87 d in PDSA cycle 1 to 4.20 d in PDSA cycle 2. XmR charts depicting TTC of all dataset are shown in Figure 1.
Discussion
This project highlights the importance of forming a multidisciplinary team for a highly complex issue that involves multiple different types of patient care providers. Collaborative efforts to improve quality of care have far-reaching impact. The team plans to continue to make improvements in the timely administration of planned inpatient chemotherapy. The team will update the Cancer Center leadership and other UVA Medical Center leadership on key findings and proposed future interventions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal